Cough Syrup Tragedy Deepens: Owner Arrested After MP Toll Hits 21
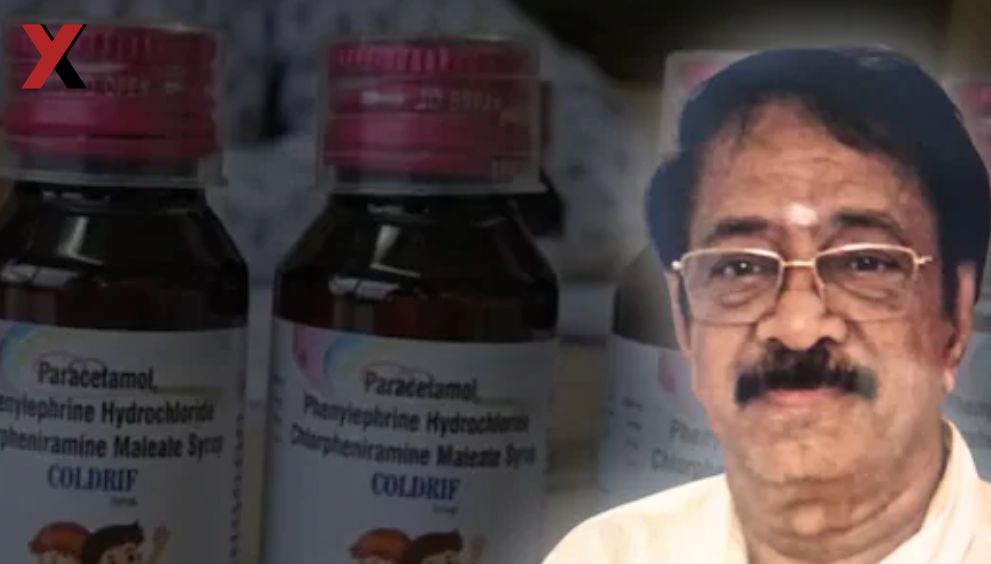
In a shocking turn, G. Ranganathan, owner of the pharmaceutical firm linked to the toxic cough syrup Coldrif, has been arrested in Chennai in connection with the deaths of at least 21 children in Madhya Pradesh. The incident has sparked widespread outrage and intensified debates over drug regulation and accountability across India.
What’s the Case?
Over recent weeks, a spate of child deaths in the Chhindwara district raised red flags. All of the victims reportedly consumed doses of Coldrif, which tests later revealed to contain diethylene glycol (DEG) — a dangerous chemical far exceeding safe limits. The deaths were characterized by acute kidney failure, leading to a rapid and tragic toll.
Authorities traced the supply chain and identified Sresan Pharmaceuticals (sometimes referred to as Srisun) as the manufacturer of the implicated syrup. After investigations and pressure from affected families and media, police forces from Madhya Pradesh coordinated with Tamil Nadu authorities and ultimately arrested Ranganathan from his residence in Chennai.
Legal Status, Charges & Company Moves
Ranganathan now faces a range of serious accusations, including:
- Culpable homicide not amounting to murder
- Drug adulteration and violations of the Drugs & Cosmetics Act
- Endangering public health and safety
Soon after the arrest, regulatory authorities moved to suspend the license of Sresan Pharmaceuticals. Plans are underway for permanent revocation of the license and sealing of the manufacturing facility, citing severe safety violations and procedural lapses.
Regulatory Oversight Under Spotlight
The tragedy has exposed alarming gaps in India’s pharmaceutical oversight. Questions are now being raised about:
- The screening of raw materials and chemicals in cough syrup production
- Enforcement of batch testing protocols
- Timely inspections and accountability mechanisms at local and national drug control bodies
In fact, the World Health Organization has flagged deficiencies in India’s testing processes, emphasizing the need for stricter regulation and monitoring of all syrup medicines marketed domestically.
Reactions & Public Sentiment
The arrest has drawn both relief and renewed anger. Families of the victims, many of whom lost toddlers, are demanding justice and compensation. Several state governments have announced monetary relief packages and initiated inquiries into local medical practitioners and dispensaries.
Medical associations have also urged caution in placing blame on prescribing doctors, pointing to systemic failures in drug safety and manufacturing oversight.
What Happens Next?
- Court proceedings & remand — Ranganathan is expected to be produced before the Chhindwara district court under tight security.
- Investigation widening — Probes will extend across the supply chain: chemical suppliers, distributors, stockists, and retailers.
- Regulatory reforms — Strengthened drug testing norms, unannounced inspections, and tighter control over high-risk formulations are anticipated.
- Compensation & support — Families will push for accountability; courts may order compensatory relief and penal measures.
- Public health response — States may launch awareness campaigns, especially in rural areas, warning against misuse of over-the-counter syrups in children.


 English
English